To Form An Ion A Sodium Atom - Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. (i) formation of sodium ion: The sodium ion, na +, has the electron configuration with an octet of electrons. A neutral sodium atom consists of 11 protons and 11 electrons. When sodium loses its outermost electron, the atom transforms. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge.
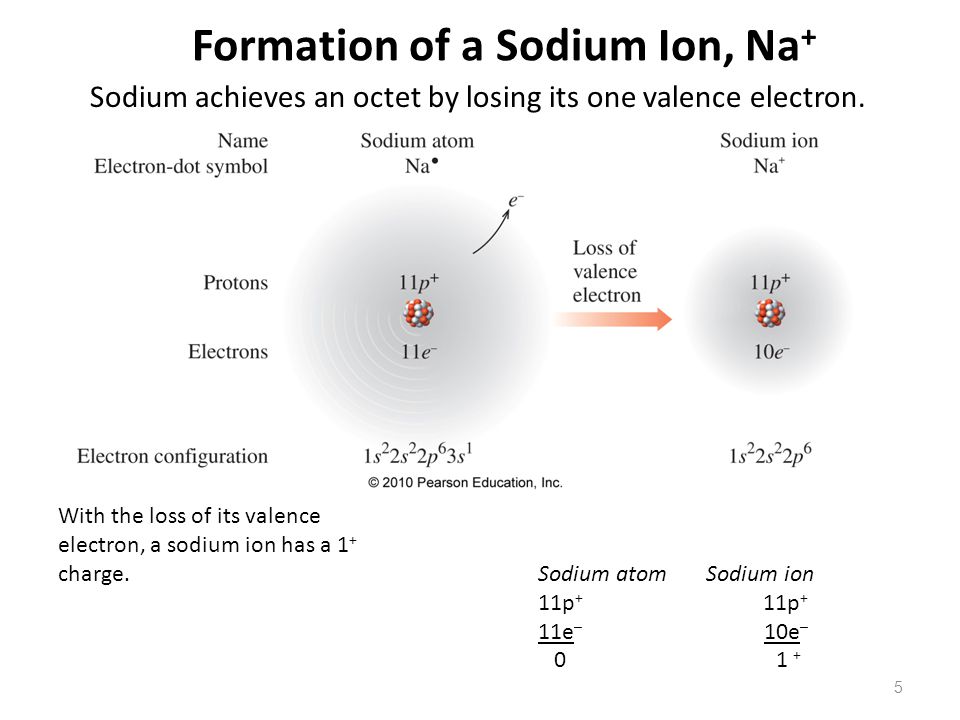
Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. (i) formation of sodium ion: The sodium ion, na +, has the electron configuration with an octet of electrons. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. A neutral sodium atom consists of 11 protons and 11 electrons. When sodium loses its outermost electron, the atom transforms. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge.
When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. (i) formation of sodium ion: Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. A neutral sodium atom consists of 11 protons and 11 electrons. When sodium loses its outermost electron, the atom transforms. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. The sodium ion, na +, has the electron configuration with an octet of electrons.
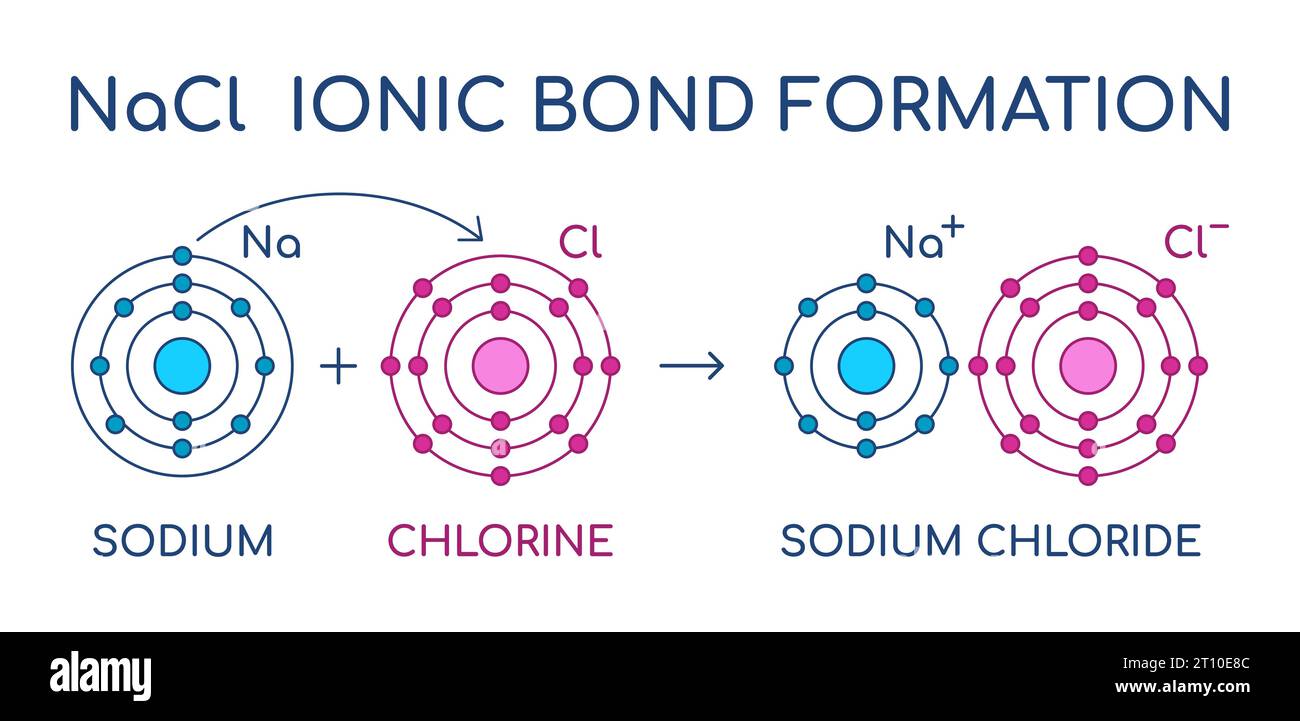
Sodium Chloride ionic bond formation. NaCl structure. Sodium and
Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. When sodium loses its outermost electron, the atom transforms. (i).
Solved Two students worked together to create a bohr model of a sodium
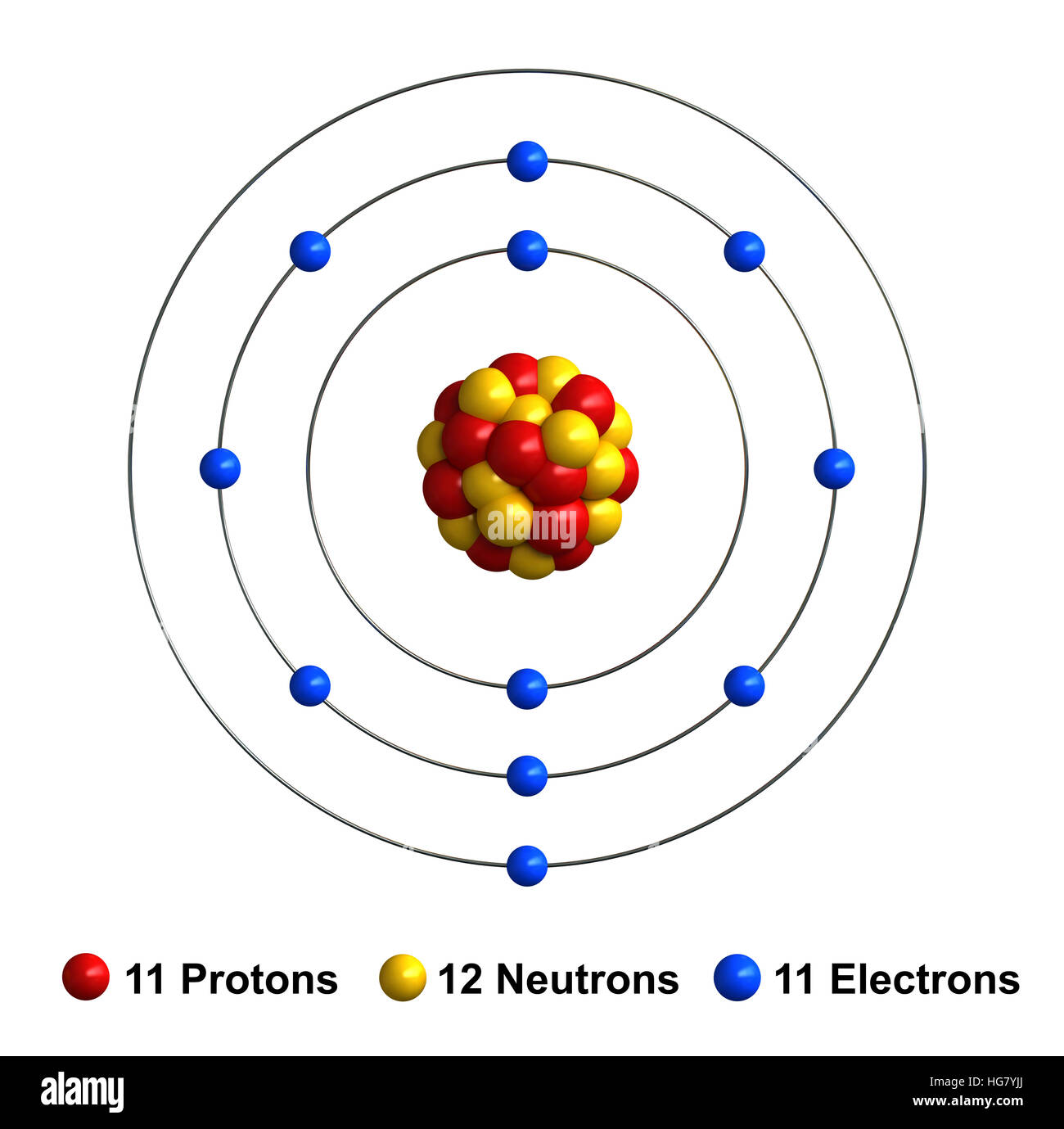
A neutral sodium atom consists of 11 protons and 11 electrons. When sodium loses its outermost electron, the atom transforms. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. (i) formation of sodium ion:
Structure Of Sodium Atom Hot Sex Picture
When sodium loses its outermost electron, the atom transforms. A neutral sodium atom consists of 11 protons and 11 electrons. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. The sodium ion, na +, has the electron configuration with an octet of electrons. Ions form when atoms lose or gain electrons close electron subatomic.
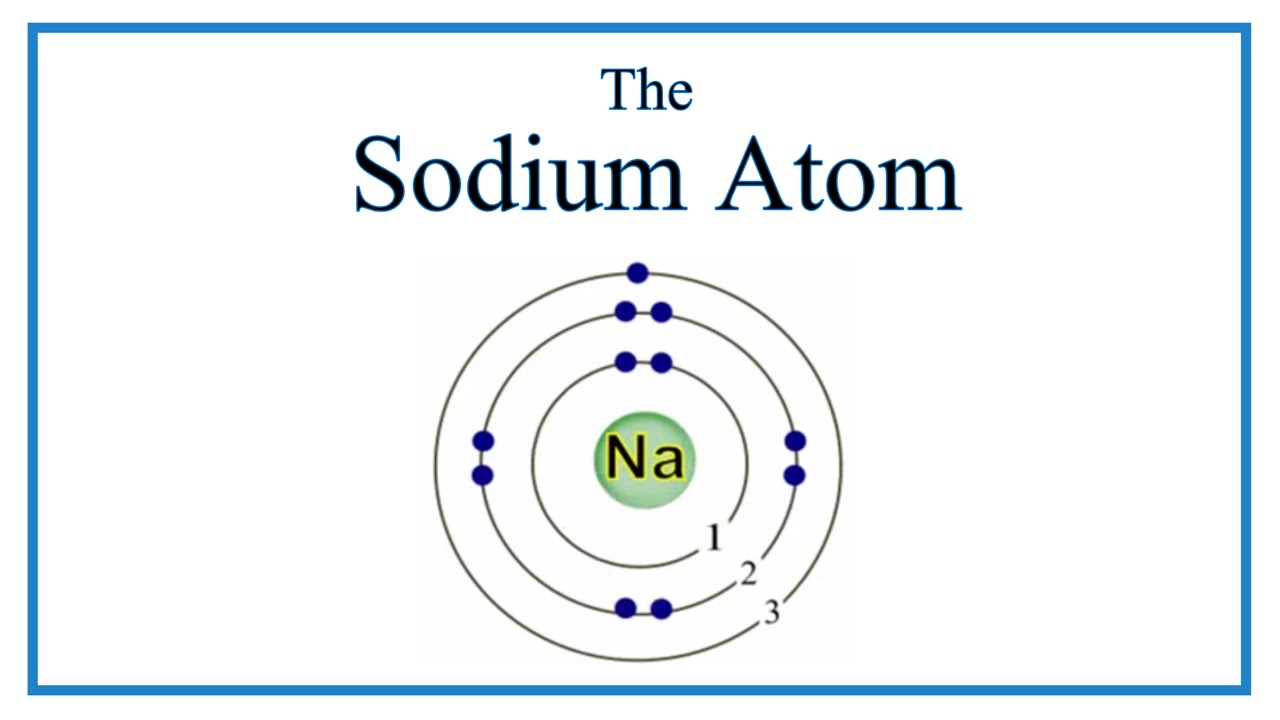
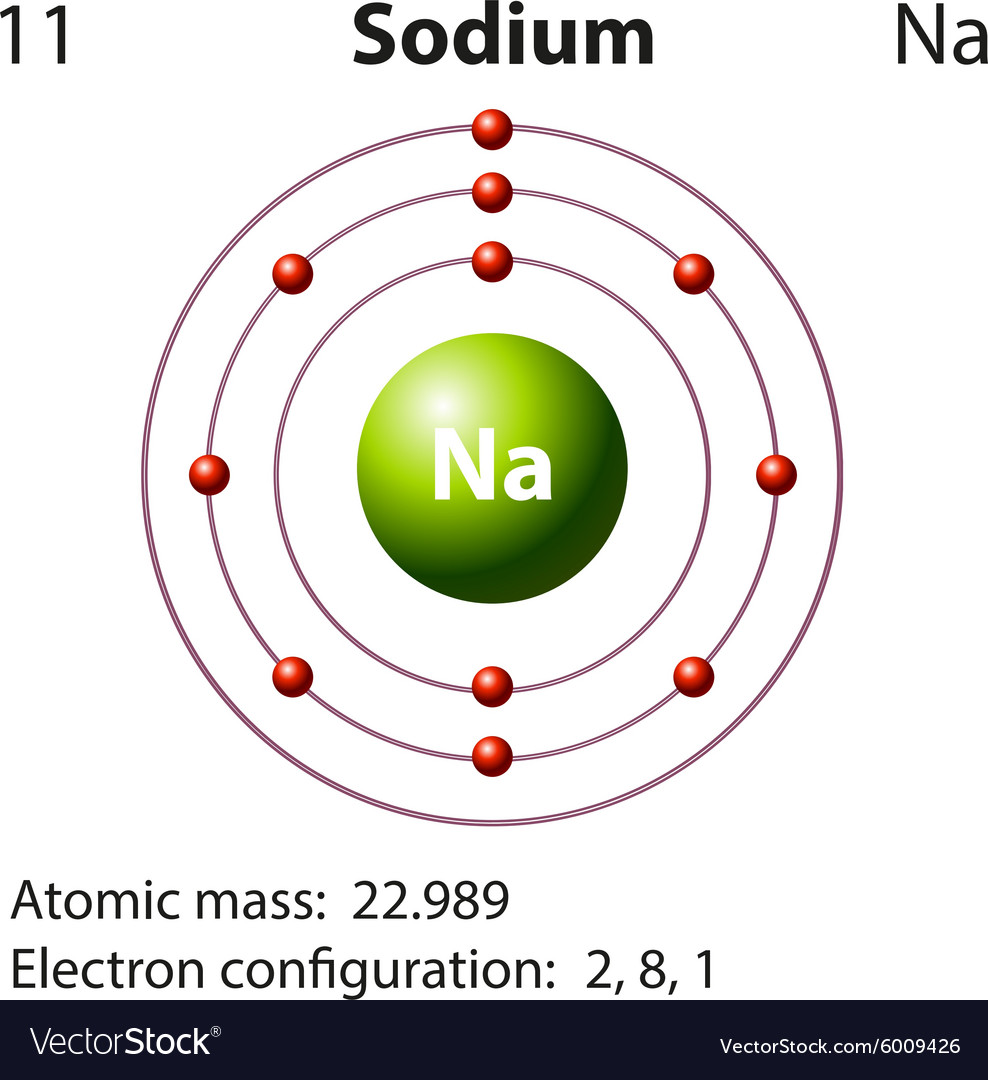
The structure of the sodium atom is shown in the figure given alongside
When sodium loses its outermost electron, the atom transforms. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. (i) formation of sodium ion: Ions form when atoms lose or gain electrons close electron subatomic.
When A Sodium Atom Reacts With A Chlorine Atom To Form A Compound The
(i) formation of sodium ion: A neutral sodium atom consists of 11 protons and 11 electrons. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. The sodium ion, na +, has the electron configuration with an octet of electrons. When sodium loses its outermost electron, the atom transforms.
Draw a diagram to show the electronic structure of a sodium Quizlet
When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. When sodium loses its outermost electron, the atom transforms. (i) formation of sodium ion: A neutral sodium atom consists of 11 protons and 11 electrons.
Formation+of+a+Sodium+Ion,+Na+
(i) formation of sodium ion: When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. A neutral sodium atom consists of 11 protons and 11 electrons. The sodium ion, na +, has the electron configuration with an octet of electrons. Ions form when atoms lose or gain electrons close electron.
How To Draw A Sodium Ion Image to u
Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. The sodium ion, na +, has the electron configuration with an octet of electrons. Ions form when atoms lose or gain electrons close electron subatomic.
Sodium Atom Bohr Model Vector Illustration 267662272
(i) formation of sodium ion: When sodium loses its outermost electron, the atom transforms. A neutral sodium atom consists of 11 protons and 11 electrons. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge.
Sodium Ion Bohr Model
Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. (i) formation of sodium ion: A neutral sodium atom consists.
The Sodium Ion, Na +, Has The Electron Configuration With An Octet Of Electrons.
(i) formation of sodium ion: When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. A neutral sodium atom consists of 11 protons and 11 electrons. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative.
Ions Form When Atoms Lose Or Gain Electrons Close Electron Subatomic Particle, With A Negative.
When sodium loses its outermost electron, the atom transforms.